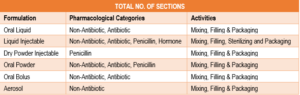
Production Sections:
Selmore’s state-of-the-art pharmaceutical manufacturing plant spreads over 166,464 SFT and has 48,744 SFT covered area. A modernized WHO-cGMP certified plant is producing high quality veterinary pharmaceutical products according to the WHO-cGMP standards to fulfill the needs of veterinarians globally. Plant is fully equipped with advanced manufacturing facilities that enable us to meet the quality protocols.
Selmore is creating new dimensions for veterinary health by offering quality products at affordable price with maximum efficacy. We are continually improving our materials, processes, equipment and human resources.
Quality Control & Microbiology Laboratories:
Quality Control Laboratory is always considered the back bone of a Company. It is equipped with latest HPLC, Spectrophotometer, Stability Chambers in short all the required instrumentation for maintain quality of international standards. Company has well-defined quality control system consisting of team of highly qualified professionals and a well-developed Quality Control Laboratory which is established for the testing, approval and rejection of starting material (active pharmaceutical ingredients and excipients), packaging components, in-process and finished products.
Microbiological Laboratory which is a subsidiary of QC Laboratory has separate sterile area, media preparation room, incubation area.
A separate sample keeping room is provide to Quality control for storage of already dispatched batches to market so that post production stability studies can be conducted.
Research & Development Process:
Research & development section is there to meet all the pre and post marketing studies, stability, efficacy etc. the department is fully operational for developing new line of molecule to be introduced in the range. Special measures are taken to check the efficacy of medicine after-market dispatches. Special arrangements are there for close intact with marketing team to check the ongoing field trials & complaint handlings.
